5 Key Elements to A Successful Digital Maturity Assessment

Part of SCW’s Journey to A Self-Driving Supply Chain
Imagine a pharmaceutical supply chain where AI-powered forecasting reduces waste, blockchain ensures drug provenance, intelligent automation streamlines operations, and real-time temperature monitoring minimizes costly cold chain failures. The first step towards achieving this vision is a digital maturity assessment. It provides a data-driven roadmap for transforming your supply chain into a competitive advantage.
#1 Defining Digital Maturity in the Pharma
Digital maturity is often used loosely, creating confusion. In the pharmaceutical supply chain, it encompasses much more than simply implementing new technologies. True digital maturity encompasses a holistic view on strategic goals and resulting transformation in how data is collected, analyzed, and used to drive decision-making, optimize processes, and foster collaboration – all within a highly regulated environment.
Key Values of Pharma Supply Chain Digital Maturity
End-to-End Visibility: Real-time data tracking across suppliers, manufacturing, distribution, and potentially to the patient level. This is essential for regulatory compliance and enabling proactive risk mitigation.
Agility: A digitally mature supply chain leverages technologies that support quick adjustments to changing regulations, market disruptions, and new product launches.
Ecosystem Collaboration: Secure, structured data exchange among supply chain partners to reduce delays and errors (example: blockchain-enabled data sharing for temperature monitoring).
Advanced Analytics: Moving beyond basic reporting to predictive and prescriptive analytics powered by AI and machine learning. (Example: AI-powered demand forecasting to reduce inventory waste and stockouts).
Process Automation: Implementing intelligent automation (beyond basic RPA) to streamline repetitive tasks, improve accuracy, and free employees for strategic work.
#2 Developing a Tailored Assessment Framework
Generic maturity models offer a starting point, but pharmaceutical supply chains demand a tailored approach. Your assessment framework must be customized to address your company-specific challenges, priorities, and your unique position within the broader pharma landscape.
Assessment questions should directly map to your company’s strategic objectives, such as:
- Cost Reduction: Are there opportunities to optimize inventory levels and reduce waste?
- Resilience: Can you quickly adapt to disruptions (supply shortages, demand spikes)?
- Compliance Risk Mitigation: Are your systems up-to-date with serialization (or other relevant) requirements?
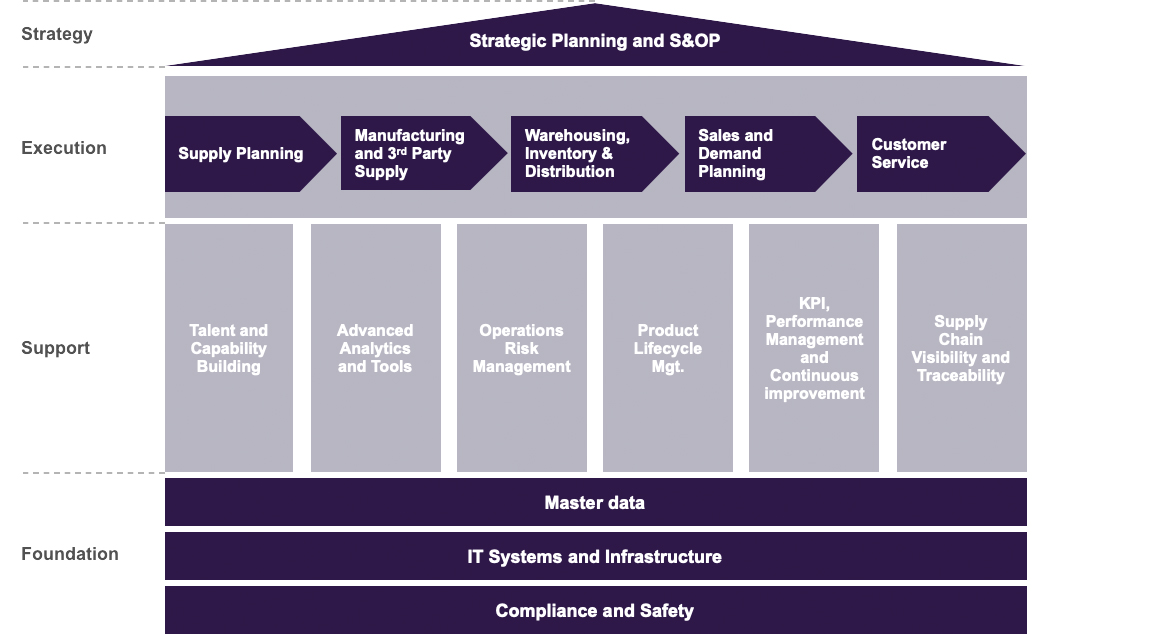
A comprehensive assessment typically focuses on these core Levels of the supply chain:
Level 1: Strategic Planning and S&OP
- Strategy & Vision
- Governance Model
- Organizational Maturity
Level 2: Supply Chain Execution
- Forecasting & Demand Planning
- Current Methods: What forecasting methods do you currently use (spreadsheets, statistical models, ERP-based planning)? Are they primarily manual or tool-assisted?
- AI/ML Potential: Are you aware of how AI & Machine Learning can enhance demand forecasting accuracy, especially under volatile market conditions? Are you open to exploring these technologies?
- Sensing Demand Signals: Do you incorporate real-time market data (point-of-sale data, social media trends, etc.) into your demand planning, or are you relying on historical data alone?
- Supply Planning
- Production Alignment: How well-integrated are your forecasting and supply planning processes? Can your supply plans quickly adjust to changes in forecasted demand?
- Constraint Management: Can your planning systems identify potential bottlenecks and proactively suggest scenarios for mitigation?
- External Integration
- Supplier Collaboration: Do you have secure data sharing mechanisms with your key suppliers? What level of visibility do you have into your suppliers’ inventory and capacity?
- Logistics Partners: Are real-time status updates on shipments shared seamlessly with your logistics providers? How is exception management handled?
- Customer Insights: Do you incorporate end-customer data (where applicable) into your demand planning and inventory management?
- Manufacturing
- Process Automation: Which production processes are still manual, and which have the potential for automation/robotics?
- Production Planning & Scheduling: How well is production planning integrated with demand and supply signals?
- Regulatory & Quality: Do you have digital systems to track batch records, quality control data, and compliance documentation?
- Packaging
- Serialization: How do you currently meet serialization requirements (if applicable)? What challenges are you facing?
- Flexibility: Can your packaging lines quickly adapt to changes in product format, volume, or labeling requirements?
- Traceability: How is traceability data for individual product units captured and linked to higher-level case/pallet data?
- Inventory Management
- Optimization: How do you currently set safety stock levels and inventory targets across various locations? Are these calculations data-driven or based on historical patterns?
- Real-time Visibility: Can you track inventory levels and expiration dates in real-time?
- Warehousing: What warehouse management systems (WMS) are in place?
- Distribution & Logistics
- Transportation Management: Do you have a TMS, or is it a transportation planning manual? What level of shipment tracking exists?
- Cold Chain Compliance: How do you monitor and document temperature excursions during transportation?
- Network Optimization: Have you regularly assessed your distribution network (number of warehouses, routes) to optimize costs?
Level 3: Supply Chain Support Pillars
- End-to-End Visibility: Can you track all your components from raw materials to finished goods in real-time across your entire supply chain (suppliers, manufacturing sites, distribution centers, etc.)? How is this information aggregated?
- Advanced Analytics: How do you currently use the data you collect? Is it mainly for basic reporting, or for advanced analysis to support decision-making? Do you use analytical tools to “what-if” scenarios, test supply chain resilience under various disruptions, or model the impact of changes? Are you leveraging AI/ML in any aspects of your supply chain analytics (forecasting, inventory optimization, risk prediction)?
- Visibility & Reporting: How is data collected, processed, and visualized across your supply chain? What level of real-time insight, predictive, and prescriptive analytics is in place?
- Risk Management: What is your current approach to risk: responding to events as they happen, or proactive identification and mitigation? Do you utilize any tools to map potential supply chain disruptions (supplier failures, geopolitical events, etc.) and assess their potential impact? How confident are you that your processes and systems can quickly adapt to new regulations, especially in areas like serialization?
- KPI & Performance Management: Do you have clearly defined KPIs across different stages of the supply chain? Are these agreed upon across various teams? How often are KPIs measured and reported? Do you have systems to automatically flag deviations from target performance? If you need to adjust KPIs due to market changes or new strategic goals, how quickly can this be done?
- Automation & Robotics Adoption: Where are you on the spectrum from manual processes to intelligent automation? What opportunities exist for streamlining operations and reducing human error risks?
- Talent / Resource Management: Do your current personnel have the skills (data analysis, change management, etc.) needed for a digitally mature supply chain? Do you have a strategy to bridge any skills gaps, either through training current employees or recruiting new talent? How do you incentivize collaboration between various teams (IT, operations, suppliers) crucial for successful digital transformation?
- Continuous Improvement Plan: Do you have a structured approach to continuous improvement (Lean, Six Sigma, or other methodology)? Is this embedded in your supply chain operations culture? When disruptions or unexpected issues occur, is there a process to analyze the root causes and prevent recurrence? Do you encourage experimentation and set aside resources for testing new technologies/approaches that could optimize your supply chain?
- Regulatory Compliance: How well equipped are you to meet evolving regulations and ensure data integrity? Do you have proactive risk mitigation measures in place?
Level 4: Supply Chain Foundation
- Master Data: Do you have a centralized master data management (MDM) system or are product, supplier, and location data spread across disparate systems? Is there a data governance process with clear ownership and quality standards? If serialization regulations apply to your products, how is master data (product codes, batch numbers, etc.) handled within your serialization platform and linked to other supply chain systems? How easy is it to securely share accurate master data with external supply chain partners (suppliers, contract manufacturers, logistics providers)?
- Data Governance: How do you ensure the accuracy and integrity of your supply chain data? Do you have protocols in place for compliance with relevant regulations? Are there processes for ensuring the accuracy, completeness, and consistency of your supply chain data (e.g., product specifications, inventory levels, shipment information)? Can you trace the origin and transformations of data as it moves through different systems and processes? Is this essential for audits and compliance? What safeguards are in place to protect sensitive supply chain data from unauthorized access or breaches, considering both cybersecurity and physical security of records?
- IT Infrastructure: What percentage of your supply chain applications are on-premises vs. cloud-based? Do you have a cloud migration strategy for any legacy systems? How well do your various supply chain systems (ERP, WMS, TMS, etc.) communicate with each other? Are there data silos impacting seamless information flow? Can your IT infrastructure handle increased data volumes, new analytics tools, or changes in transaction volume without performance issues?
- System Landscape Maturity: How frequently are your systems updated with the latest security patches and software fixes? Are there outdated systems posing an operational or compliance risk? Do you have a plan for modernizing your IT landscape to support increased digitalization of your supply chain? Is this plan aligned with your business objectives and anticipated future needs? How do you evaluate and select technology vendors? What processes exist for managing vendor relationships, especially for mission-critical systems?
Developing a nuanced assessment framework aligned with your business goals often benefits from external expertise. Consultants like Supply Chain Wizard specialize in digital transformations specifically within the pharmaceutical industry. Supply Chain Wizard leverages a proprietary maturity assessment tool, refined through numerous client engagements, to provide actionable insights and accelerate your digital roadmap development. Ready to assess your pharma supply chain’s digital maturity? Contact us for a consultation.
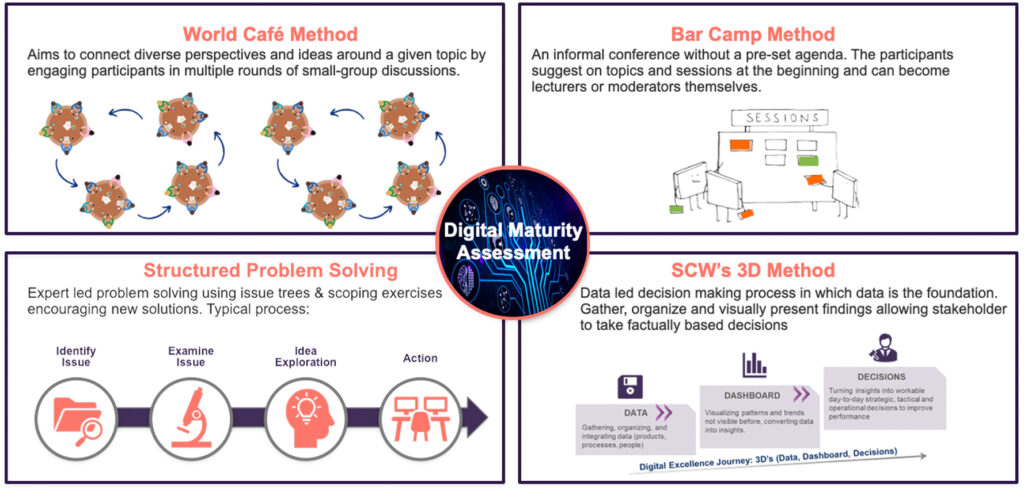
#3: Choosing the Right Assessment Methodology
Sailing the digital transformation seas requires the right navigational tools. In this case, the “ship” is your pharmaceutical supply chain, and the “tools” are the various digital maturity assessment methodologies available. Numerous digital maturity models exist, each with its strengths and weaknesses. Some models are stage-based, categorizing companies into levels like “Nascent,” “Emerging,” or “Connected.” Others, like the CMMI model, focus on process capability.
Ultimately, the choice of methodology depends on your company’s specific needs and resources. Here are key factors to ponder:
- Company Size & Complexity: For a smaller pharma company with a streamlined supply chain, a simpler stage-based model might suffice. Larger, more complex organizations might require a more nuanced approach.
- Available Resources: Conducting a comprehensive assessment can be resource-intensive. Consider the time and personnel you can dedicate to the process.
- Desired Outcomes: Are you aiming for a high-level overview or a deep dive into specific areas of your supply chain?
Often, the most effective approach is a hybrid one. You could leverage a stage-based model for a general framework and then supplement it with targeted assessments in critical areas (e.g., cold chain logistics compliance).
Choosing the right methodology can be a complex endeavor. Supply Chain Wizard’s consultants have extensive experience applying various models within the pharma industry. We’ll work with you to select the approach best suited to your unique goals.
#4: Building a Roadmap Based on Assessment Results
The digital maturity assessment serves as a diagnostic tool, pinpointing strengths and illuminating areas for focused digital investment within your pharmaceutical supply chain. To realize the full potential of this analysis, a clear, actionable roadmap is imperative.
Assessment results will likely reveal a range of potential digital initiatives. Strategic prioritization is crucial for optimizing resource allocation and ensuring timely ROI. Consider the following:
- Alignment with Strategic Imperatives: Prioritize initiatives that directly support your most critical business objectives (cost reduction, resilience, compliance risk reduction).
- Feasibility Assessment: Balance transformative aspirations with a realistic assessment of budget constraints, available expertise, and project timelines.
- Risk Mitigation: Prioritize areas posing significant risks to compliance or operational continuity if not addressed promptly.
Embrace a measured, multi-phase roadmap to maintain operational stability while driving continuous improvement. Each phase should have well-defined objectives, measurable KPIs, and allocated ownership.
Technology adoption hinges on people. Embed a comprehensive change management plan within your roadmap. Proactive communication, targeted training, and continuous stakeholder engagement ensure employee readiness and acceptance.
Focusing on low effort and high business impact activities can result in rather short projects with high benefits which are often referred to as the quick wins. One example is evaluating shorter, often very repetitive and very standard process steps, in example gathering data for monthly meetings. If deemed eligible, implementing robotic automation can turn a 30 minute activity into a 30 second activity, raising productivity of your team 20 fold in less than 3 months.
#5: Partnering with Experts for Success
While a digital maturity assessment can be conducted in-house, partnering with experienced consultants often maximizes the value and expedites the transformation process. Here’s why:
- Specialized Expertise: Consultants like those at Supply Chain Wizard bring deep knowledge of both digital technologies and the unique complexities of pharmaceutical supply chains. This combination ensures that your assessment and subsequent roadmap are grounded in both technical best practices and industry-specific realities.
- A Fresh Perspective: Internal teams can sometimes become blind to inefficiencies embedded in long-established processes. External consultants provide an objective lens, identifying potential improvements hidden in plain sight.
- Best Practice Benchmarks: Seasoned consultants have conducted assessments for numerous pharma companies. They can benchmark your organization’s digital maturity against industry standards, helping you set realistic goals and timelines.
- Accelerated Transformation: Leveraging a consultant’s proven assessment frameworks and change management expertise streamlines the process. You’ll navigate the roadmap from insights to implementation faster.
- Collaborative Approach: The best consultants view the assessment as a partnership. At Supply Chain Wizard, we work closely with your internal stakeholders, ensuring knowledge transfer and empowering your team to become champions of digital transformation.
For additional detail and help with Digital Maturity Assessment, please contact:
Mia Van Allen – Managing Partner – mia.vanallen@supplychainwizard.com
Our expertise is not generic. We focus specifically on driving successful digital transformations within the pharmaceutical industry. Our assessment framework, sharpened through numerous client engagements, delivers actionable insights aligned with the most pressing challenges facing pharma supply chains. Our goal is not just to deliver a report but to guide the implementation of a roadmap that delivers a measurable impact on your bottom line, patient safety, and the efficiency of your supply chain.
The time for speculation is over. A digital maturity assessment empowers you with the knowledge needed to make informed choices about transforming your pharmaceutical supply chain. If you’re ready to turn this knowledge into action and build a future-proof supply chain, Supply Chain Wizard is here to guide your journey.
For more information about SCW Consultancy Services;

