Whitepaper:
Streamlining Alert Management: Integrating EMVO’s AMS with RPA Integration
Executive Summary
Pharmaceutical serialization compliance poses significant operational challenges, particularly across multiple regulatory environments such as the European Medicines Verification Organization’s Alert Management System (EMVO AMS). Traditionally manual alert management processes risk regulatory compliance, operational efficiency, and scalability.
SCW Consulting leverages over 15 years of dedicated expertise in serialization and regulatory compliance to introduce a modular AMS-RPA integration solution. This robust integration strategically utilizes Robotic Process Automation (RPA) technology to create an agile, automated, and hands-on serialization management system, directly addressing alert management complexities.
Current Challenges in Alert Management
Manual Processes and Resource Constraints
Pharmaceutical companies today face substantial manual workloads when managing alerts related to serialized medicines through EMVO’s AMS. Tasks such as data extraction, stakeholder notification, action logging, and status updating consume considerable employee time and resources.
Lack of Standardization and High Error Rates
A lack of standardized processes across affiliates leads to inconsistent handling, potential compliance breaches, and operational inefficiencies. Manual data handling amplifies the likelihood of errors, increasing compliance risk and hindering smooth operations.
Limited Scalability
Existing manual processes fail to scale efficiently to handle increasing alert volumes or adapt to evolving regulatory requirements, jeopardizing compliance and operational sustainability.
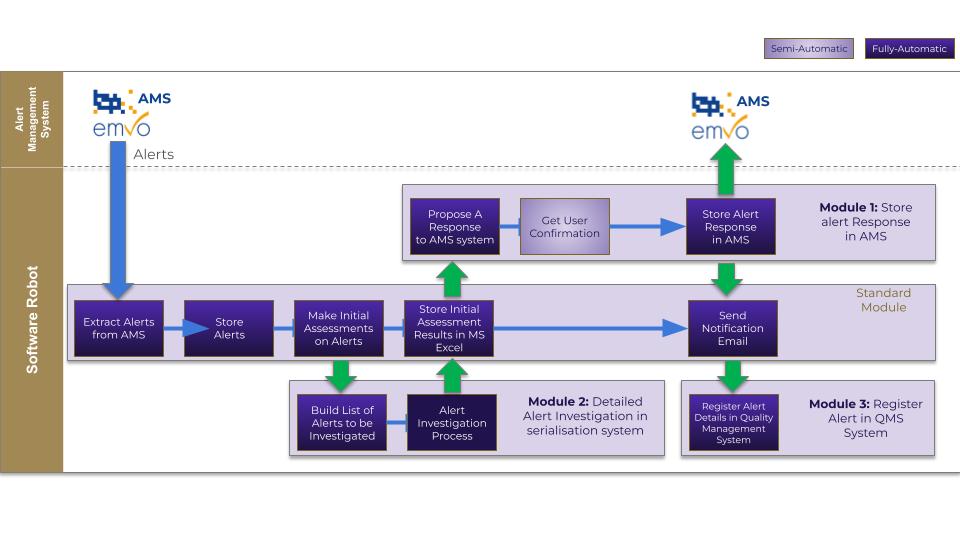
SCW Consulting’s Modular AMS-RPA Solution
Automated Alert Management
SCW Consulting’s modular solution leverages RPA to automate critical aspects of alert management. Utilizing tools such as UiPath integrated seamlessly with AMS, our solution provides real-time data processing, streamlined alert triage, and standardized workflows, significantly reducing manual interventions.
Flexible Modular Design
Our modular architecture is fully customizable for customer requests, enabling tailored implementations that align with each client’s specific operational needs and regulatory requirements—driving maximum efficiency and ensuring steadfast compliance.
Enhanced Compliance and Reduced Risks
Automated, standardized workflows ensure alerts are addressed promptly, consistently, and accurately, minimizing compliance risks and ensuring robust audit trails for regulatory purposes.
Key Functional Components
- Data Extraction and Reporting: Automatically extracts data from AMS, generating daily, weekly, monthly, country-wise, and technical trend reports.
- Proactive Alert Management: Leverages agentic AI to update AMS alert statuses based on predefined business rules and escalate issues in real time.
- Customizable Reporting Framework: Provides customized reports aligned precisely to client expectations and formats.
- Cross-Platform Integration: Registers alert statuses across multiple platforms, including deviation, complaint, and quality management systems (QMS).
Real-World Results and Benefits
Operational Efficiency Improvement
Automation of routine tasks saves substantial employee hours each month, allowing teams to focus on strategic and analytical responsibilities, significantly enhancing productivity.
Compliance Accuracy
RPA-driven processes ensure uniform adherence to regulatory requirements, substantially reducing compliance risks related to human error.
Scalability and Agility
The solution provides a scalable framework, supporting seamless expansion across associated entities, facilitating operational continuity, and adapting effortlessly to evolving regulatory landscapes.
Enhanced Visibility
Comprehensive dashboards offer real-time monitoring and detailed insights into alert management activities, empowering decision-makers with actionable data.
Case Study Highlight
In a recent implementation for a global pharmaceutical client, SCW Consulting’s AMS-RPA integration solution achieved significant outcomes:
- Reduced investigation response time by automating alert triage and management.
- 25+ hours saved monthly, allowing for increased focus on high-value tasks.
- Standardized processes across multiple platforms, enhancing overall operational efficiency.
- Accelerated compliance responses to eliminate compliance risks and minimize supply chain disruptions.
Conclusion: The Path Forward
SCW Consulting’s AMS-RPA integrated modular solution marks a pivotal advancement in serialization management, offering pharmaceutical companies a robust framework for future-ready serialization compliance. With hands-on management capabilities powered by automation and expert guidance, organizations achieve increased operational efficiency, regulatory compliance, and scalable growth.
Partner with SCW Consulting today to harness over 15 years of serialization expertise and secure your supply chain’s future with state-of-the-art AMS-RPA integration.
For more information about SCW Consultancy Services;
For additional detail and help with AMS-RPA Integration, please contact:
Yigiter Colakoglu – Managing Partner – yigiter.colakoglu@supplychainwizard.com

